Introduction:Adult T cell Leukemia/Lymphoma (ATLL) is a mature T-Cell-neoplasm related to human T-cell lymphotropic virus type 1 (HTLV-1) infection that shows variable clinical presentation and adverse prognosis with shorter overall survival (OS) when compared to other peripheral T-cell lymphomas (PTCL). Previous epidemiological studies estimated cumulative incidence in endemic regions around the world. In Brazil, mainly due to its vast dimension, data collection has limitations and is subject to bias. In April 2015 theBrazilianT-cell longitudinal project initiative was launched. One of the primary purposes was the collection of epidemiological and clinical data from the most frequent subtypes of newly diagnosed PTCL. Among them, 41 cases of ATLL were recorded.
Objectives:The aim of this study is to describe clinical features, frequency and overall survival (OS) of 41 cases of ATLL registered in the ongoingBrazilianT-Cell Project.
Methods:This is an ambispective observational study design collecting baseline characteristics, clinical features including date of diagnosis, clinical subtypes, B symptoms, performance status, Ann-Arbor staging, HTLV-1 status, number of sites, nodal and extra nodal presentation and types of skin lesions, peripheral blood counts and biochemical tests, front-line treatment and best response after first-line treatment. REDcap Platform has been used to collect and store data and for descriptive analysis the IBM-SPSS version 24 was applied. Kaplan-Meier method estimated the OS, whereas Log-Rank tests to compare its curves. OS period was calculated from diagnosis date until death or last seen date, and event was death by any cause.
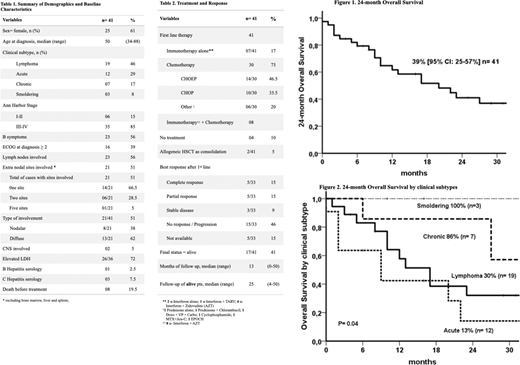
Results:Out of 281 cases of PTCL registered so far, 41 were ATLL cases. The median age was 50 years (34-88), 25 (64%) female; a higher incidence of lymphoma subtype was observed (46%), followed by acute (29%), chronic (17%) and smoldering (8%). Most of the patients (85%) had advanced-stage disease (III-IV, Ann-Arbor) and 56% had B symptoms (Table 1); 73% received chemotherapy with anthracycline-based regimens (46.5% CHOEP; 33.5% CHOP; 20% others) whereas 17% were managed with immunotherapy and antiviral therapy. The overall response rate was 30%; no response or progression 46%; stable disease 9% and 15% no data available yet (Table 2). Median follow-up was 13 months and 25 months for 41% of alive patients. Two year OS was 39% (95%CI: 23-55%) (Figure 1). Smoldering and Chronic subtypes showed better OS when compared with acute and lymphoma (100% smoldering, 86% chronic; 30% lymphoma type and 13% acuteP=0.04) (Figure 2). The multivariate Cox Regression analysis found male gender (HR 10.9 95%CI: 3.0-39.7, P<0.0001) and albumin (HR 0.23 Beta -1.45 95%CI: 0.10-0.55, P=0.001) as significant prognostic factors for OS.
Discussion:ATLL prognosis remains poor regardless of the type of treatment regimen and may be associated with the high incidence of lymphoma and acute subtypes, as well as advanced stage disease presentation. Despite the high number of cases seen in southeastern region of Brazil, it is important to emphasize there are still limited data from other regions. Albeit the sample size is small, these findings confirmed literature review data so far, with poor overall survival in a short time and gender and albumin as predictors of OS in the multivariate analysis.
Conclusion:This study highlights the poor prognosis associated with ATLL. Moreover, it seems relevant to expand this study to all Brazilian regions. Hence, the need for early diagnosis and new treatment strategies, able to reduce mortality is warranted, that could possibly change the nature and spectrum of disease.
Federico:Spectrum:Consultancy, Membership on an entity's Board of Directors or advisory committees;Sandoz:Consultancy, Membership on an entity's Board of Directors or advisory committees;Cephalon/Teva:Research Funding;Mundipharma s.r.l.:Research Funding;Celgene:Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding;Millennium/Takeda:Research Funding;Roche:Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding;Takeda:Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal